|
|
|
Cancer: n. 1. any type of malignant growth or tumour, caused by abnormal and uncontrolled cell division. 2. the condition resulting from this. 3. an evil influence that spreads dangerously.1
Cancer is a broad term describing a diverse group of diseases sharing the common characteristic of abnormal cellular division that is not subject to normal growth controls. It is one of the most feared diseases due to a general perception that it is an indiscriminate and incurable affliction that insidiously attacks people of all cultures and ages. While cancer is an ancient disease, the inability to cure cancer has persisted despite rapid increases in social and economic improvements and impressive advances in scientific knowledge and medical techniques. The perception that the rate of cancer infections is increasing has recently been a matter for public discussion as has the recognition that the incidence of cancer is related to exposure to modern day chemicals. The western world has virtually eliminated a large number of "incurable" infectious diseases in the past, such as polio, scarlet fever, tuberculosis, diphtheria and typhoid fever, but the cure for cancer remains stubbornly elusive.
It is the western world that appears to be most affected by cancer (Table 1-1).
|
Country |
Males |
Females |
|
USA |
2578 |
2200 |
|
England and Wales |
2545 |
1963 |
|
Germany |
2444 |
2166 |
|
Denmark |
2221 |
2000 |
|
New Zealand (Europeans) |
2421 |
2233 |
|
Nigeria |
767 |
1048 |
Number of persons certified as afflicted per million
It is difficult to make absolute comparisons of the levels of cancer between countries because of the differences in the quality of medical services and less accurate diagnosis and registration, however, it becomes clear from Table 1-1 that the incidence of cancer shows curious differences with geographic variation. Nigerian men are relatively unlikely to die from cancer, whereas their wives are at significantly greater risk. This is the opposite to the trend shown for western societies in which men are much more susceptible to cancer than women and both groups are much more likely to be afflicted than Nigerians.
Many studies have been reported3 that correlate local customs, diet and lifestyle with the onset of cancer, for example the relationship between the per capita consumption of meat (or conversely the low consumption of cereal, which tend to occur together) and the rate of cancer of the digestive tract. Countries of low red-meat consumption have lower levels of cancer of the large intestine, whereas societies with a large intake of meat, such as New Zealand, USA and Canada have significantly larger annual incidences of the disease. It is believed that such a diet favours the growth of certain bacteria capable of converting the bile normally present in the bowel into carcinogens. The diet of African natives is largely based on foods with high vegetable fibre content which pass through the bowel much more quickly than a typical western diet, laced with animal proteins. Constipation, frequent in westerners, is symptomatic of the slow transit of food through the bowel and results in prolonged exposure to the bacterially generated carcinogens. Clearly the incidence of cancer is, to a significant degree, determined by diet.3,4
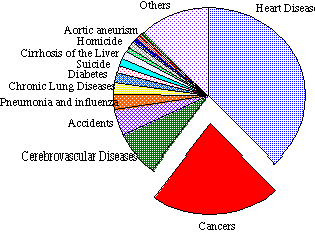
Cancer is the second most prolific disease in the United States of America.

Second only to heart disease as the number one cause of death, one in every five people in the United States of America will die from cancer or the complications associated with its treatment (Figure 1-1). Only last century, the major causes of most mortalities were infectious diseases, but this changed with the advent of improved antibiotics, vaccines and methods for storing and preserving foods, resulting in longer lifespan. Today, death has been confined to the aged and it is these people that are most conspicuously at risk in developing cancer. Aging populations, particularly in modern societies, have contributed to the proportional rise in degenerative disease, such as cancer and arterial diseases, and various models have been proposed to account for the clustering of cancer in the aged. One such model of carcinogenesis proposes that each cell has several genes that independently restrain it from forming a cancer and it will not do so until each of these genes has been inactivated by mutation. The probability that a cell or its ancestors has undergone externally induced mutations is proportional to the age of the organism. Indeed, the logarithm of the death rate due to cancer for US citizens has been found to be linearly related to the logarithm of age, indicating that several mutations are required to generate cancer.6 This study has important implications as it indicates that each cancer is the end result of several mutations, which are determined by age, and the history of exposure to occupational and cultural carcinogenic agents. However, knowledge of the relationship between age and death rate does not reveal what is the causative agent responsible for cancer. Many studies have investigated the relationships between age, location, and environmental factors with the incidence of cancer.2,5,6 This knowledge has pinpointed specific combinations of lifestyle and geography in which the incidence of cancer has elevated levels and helped to define the trends in the type of cancers and the degree to which they afflict various races of people.
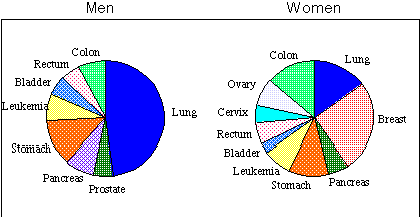
Approximately half of all cancer deaths result from cancers of four major body components: the lung, large intestine, stomach and the breast.
Breast cancer is the most common form of malignant disease in women and its frequency is rising. In 1911 the death rate was 198 per million living, rising to 415 by 1966 and in 1975 was 461. The extreme and often psychologically damaging nature of mastectomies has resulted in intense public interest in the areas of preventative and basic research. In Australia the increased awareness of breast cancer has resulted in mobile mammography units, routinely testing women for the early stages of the cancer. Other preventative methods for avoiding cancer are also being developed.
A major factor in the incidence of lung cancer can be attributed to smoking tobacco. In Australia, education is being used to encourage people to reduce the levels of tobacco consumption and prevent the expensive and avoidable demands on hospitalisation and medical care for cancer suffers. Tobacco is considered the most important known carcinogen to humans and remains the largest single avoidable cause of premature death internationally.3
Examination of the data for the death rate from cancer of the stomach shows curious changes and geographical variations. Recent advances in diagnosis and treatment have reversed the trend of an increase in these cancer rates since recorded measurements have been available. The death rate amongst men rose from the 1911 rate of 201 (per million) to 378 in 1951, but has since fallen steadily to 291. A similar peak followed by reduction was observed for women. However geographical anomalies remain common. For example cancer of the stomach is especially common in Japan where the rate of incidence is about 950 per million and interestingly, immigrants to Japan subsequently display a corresponding increase in the rate of cancer comparable to the indigenous population, indicating that an environmental carcinogen and not genetic factors may be responsible.
Cancer of the bladder occurs twice as commonly in men than in women and the death rate has been steadily rising over the last 50 years. It is generally believed that an external carcinogen is responsible for the increase, as the potentially carcinogenic waste products to be excreted are concentrated in the kidney and these could act on the mucous membrane of the bladder. The association between chemical carcinogens and cancer, particularly in relation to aromatic amines is discussed in Section 1.2 - Chemical Carcinogens.
There are over 100 different cancers. The four major types of cancer are classified7 as follows:
Carcinogens are the agents that cause cancer and can be classified in three groups; physical, viral and chemical.
Physical carcinogens include hard and soft X-rays, UV light, asbestos fibres and many other external agents.
Viral carcinogens, which for decades were considered not to cause cancer but simply take advantage of a weakened cell, have been positively linked to the onset of specific cancers.4 Birkett's lymphoma, among Africans in regions of high malaria, and nasopharyngeal carcinoma in South East China have been found to be caused by the Epstein-Barr virus.8 Cervical cancer is also believed to be associated with the Herpes Simplex Virus Type II, and certain leukemias and lymphomas are caused by the Human T-cell Leukemia virus.8
Chemical carcinogens are believed to be by far the greatest cause of cancer. They are substances that interact directly or indirectly with DNA, causing changes in the genetic code.
The metabolic mechanisms leading to the onset of cancer are extremely complex and it is generally believed that unhindered cell division is brought about by genetic mutation within the cell DNA. The process leads to an alteration in the control of protein synthesis and the change is hereditary.
Growth factors and hormones are natural chemicals that bind to receptors on the cell wall and stimulate the biochemical chain reactions that result in cell growth and division. Damaged stem cells which undergo transformation become capable of prolific growth and division. These cells do not fully differentiate and, consequently, they neither attain the particular properties nor perform the specialised functions of the cells from which they are derived. Cancer cells are usually more motile than other cells and do not have a tendency to adhere to other cells.9
A benign tumour forms if the growth of cells is physically retarded by formation of a fibrous capsule that localises the effects of the cancer cells and limits disruption of the general metabolism of the body. Benign tumours are not generally life-threatening regardless of size, however benign brain tumours can cause death due to the build up of pressure in the skull cavity and the risks of the associated surgery.
Metastasis is the process by which cancerous cells disseminate though the body after penetrating the walls of blood or lymphatic vessels. Transport to other areas of the body results in establishment of secondary tumours that can be hard to diagnose and treat by surgery or chemotherapy. Aggressive malignant cancers are frequently the cause of death.
While the best treatment is early detection, conventional cancer treatment usually includes a combination of surgery, chemotherapy and radiation therapy.
Chemotherapy involves treating the patient with antitumour drugs which destroy cells during the process of cell division and DNA replication. These drugs are therefore most effective with younger tumours, in which 30-100% of the cells are undergoing mitosis and so early detection increases the chances of survival. As the antitumour drugs do not distinguish between cancerous and normal rapid rates of cell division (eg. cells in the hair root and gastrointestinal tract), chemotherapy has severe negative side effects, such as loss of hair. Chemotherapy is therefore usually attempted over short periods followed by rest periods allowing the normal tissue to recover.
Radiation therapy, or radiotherapy, is the treatment of cancer cells with high energy, short wave radiation. This technique depends on cancerous cells being more susceptible to the effects of radiation than normal tissue. While some tumours respond to treatment it does not generally cure patients of the disease. The side effects include nausea and skin reaction, loss of hair and diarrhoea.
These conventional treatments offer an overall success rate of surviving cancer of about 50%, which increases if diagnosis occurs early. This overall rate has increased over the period of recorded measurements (1959 39%) as general medical facilities and techniques are improved and refined.
Some other experimental techniques and drugs are being investigated, such as the use of interferon and interleukin-2 to strengthen the immune system, and the use of monoclonal antibodies9 to search and destroy cancerous cells.
A chemical carcinogen is a substance that significantly increases the yield of malignant neoplasms through direct or indirect interactions with DNA. The occurrence of many human cancers appears to be determined by environmental factors, indeed many carcinogens are introduced into the environment by human activities and as such are in principle removable or at least controllable. The historical background to cancer research has clearly indicated that minimisation of exposure to chemical carcinogens is the only prevention for many cancers.
The association between the environment and the occurrence of particular kinds of cancer were first recognised when Dr John Hill, a London physician, published his conclusion on the relationship between nasal cancer and the use of snuff in 1761.10 In 1775, another British physician and surgeon, Percivall Pott, reported a connection between scrotal cancer and men who had been chimney-sweeps. Potts remarked...
"...The fate of these people is particularly hard...they are thrust up narrow, sometimes hot chimneys where they are bruised, burned and almost suffocated; and when they get to puberty, become particularly liable to a most noisome, painful, and fatal disease...of the scrotum and testicles."11
Potts noted that soot lodged in the folds of the scrotum of the boys was most possibly the cause of the disease and that operative treatment was not successful if carried out after the tumour spread. It is generally considered that carcinogens from the incompletely burned soot dissolved in the naturals oils of the scrotal skin, causing the "soot-wart" cancers. While Potts correctly surmised that cancer was "...a disease of the habit", it was the Danish and not the English that first acted on the results. Danish chimney sweeps were required to wear protective clothing and wash regularly, while the British continued with their weekly bath and the occurrence of soot-wart cancers continued for many years. The levels of scrotal cancer in Danish chimney sweeps was reduced.
The link between an occupational cancer and the probable causal agent had been clearly defined, but the lessons are still ignored even today. Cancers amongst coal-tar and shale-oil workers are similar to the cancers observed in chimney sweeps 200 years ago. Coke-oven workers in the steel industry have been found to have fatal lung cancers an order of magnitude higher than steel workers not involved with coke ovens.
In 1822, Ayrton-Paris surmised12 that arsenic fumes might be the cause of the frequent occurrence of scrotal cancer in the copper and tin smelter workers in Cromwell, England and while the use of arsenic has been reduced, large numbers of people continue to be exposed to similar arsenic compounds. Higher rates of cancers of the skin, lung, lymphatic system and liver have been found in these workers. In the following decades, further reports were released indicating that occupational exposure to chemicals was responsible for specific cancers. Von Volkmann13 found that paraffin workers in Saxony reported a high incidence of skin tumours, and in 1876, Bell14 suggested that shale oil was responsible for certain skin cancers.
These reports, which mainly emanated from research in England, were by all accounts models of lucidity but it was not until 1920 that it was made compulsory to notify the government's Chief Inspector of Factories of any incidence of occupational cutaneous cancer. It was not until this action was taken that the full extent of disease could be monitored. Unfortunately, the accuracy of the figures in the early years (pre 1960) have been questioned. Government intervention into the safety of work environments has escalated in parallel with the increase in all aspects of governmental controls from the beginning of the twentieth century.
The end of the 19th century and the start of the 20th century witnessed the publication of a number of reports on the occurrence of lesions in various occupations. The rapid industrialisation of the English economy was directly responsible for the rapid increase in number and types of cancers. The fledgling English dye industry, which was entering a growth phase was a typical industry that was beginning to flourish with the increase in world trade. Many people flocked to the new industry which essentially operated without government influence or regard to workers safety. By today's standards, obviously dangerous work practises exposed workers to large concentrations of the dyes which resulted in cancer of the scrotum and bladder.
In 1946, Henry,15 concerned with the obvious although sporadic ad hoc correlations between cancer and the workplace, undertook a detailed study of the governmental data to obtain accurate figures in relation to cutaneous scrotal cancers. His research indicated that the annual rate of incidence was 4.2 per million and, as expected, certain types of work carried a significantly greater risk in developing fatal cancers. The annual rate of cancer per million for several occupations was reported: chimney sweeps 755, patent-fuel workers 504, sack spinners 423 and workers in tar distilleries 213. These compared with the rates for: commercial travellers 0.3, butchers 0.5 and automobile drivers 0.3. Henry speculated that scrotal cancer was entirely chemically induced.
The following year, Henry16 expanded his report on cutaneous cancer of occupational origin, including the important observation that certain substances could induce cancer irrespective of the site of exposure on the body. He was however not the first scientist to make this observation. Four decades earlier, Yamagiwa and Ichikawa17 reported chemically induced tumours, in which they observed papillomas and carcinomas after applying coal-tar to the ears of rabbits.
For the pioneer cancer researchers, identification of the active chemical in the applied mixture was extremely difficult, not least because the chemistry profession was in its infancy, but also because of the complex nature of the applied mixtures. Nevertheless, research continued with studies by Hueper,18-20 Leitch21, Passey22 and others23-26 also reporting the observation of chemically induced skin tumours. It soon became clear that a large number of biologically active chemical were present in oils, tars, soots and pitches and research focussed on isolation, purification and the carcinogenic activity of the chemicals in these mixtures.27
Researchers focussed on linking chemical compounds with specific cancers and elucidating the mode of action of these carcinogens.
The carcinogenic activity of polycyclic aromatic hydrocarbons (PAH's) was first reported by Yamagiwa and Ichikawa and following from this pioneering work, Kennaway and Hieger28 tested dibenz[a,h]anthracene 1 in mice and identified it as the first pure chemical carcinogen.
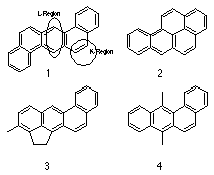
Hieger4 followed this work by purifying two tons of gas-works pitch to obtain benzo[a]pyrene 2, which was found to be carcinogenic and is recognised as the first industrially generated chemical carcinogen.31
Extensive research into the identification and effects of occupational exposure to potent polycyclic aromatic hydrocarbon carcinogens,4 such as 3-methylchlolanthracene 3 and 7,12-dimethylbenz[a]anthracene 4, was undertaken by scientists in England28 and in the United States of America.32-36
Attempts to correlate chemical structure with carcinogenic activity proved difficult but a common thread in these compounds was the benzo[a]anthracene skeleton.
Carcinogenic activity was empirically linked with several electronic features such as: alternating double bonds delocalised over the skeleton of the molecule; the presence of an active L region, typically the 1,4 locus of anthracene; and an active K region (Figure 1-3), typified by 9,10 localised C=C of phenanthrene. The historical view of the K region, in which an alternating hydrocarbon possesses a meso-phenanthrenic double bond, which is particularly susceptible to addition reactions has since been expanded to refer to any bond in a conjugated molecule which is particularly susceptible to addition reactions.
Coulson37 approached the reactivity by comparing the ¹-electron densities of various anthracene related compounds with their apparent carcinogenic activity. Coulson combined the characteristics of a K-region, and an L-region and investigated the resulting changes to the carcinogenic activity when substituents present on the ring exerted electronic demands on the K region. For example, hydroxy groups present on a polycyclic aromatic ring generally decrease the carcinogenic power. It has been suggested by Daudel and Daudel38 that the decrease in activity is probably due to an increase in the water solubility of the hydroxylated PAH's.
A correlation of the activity of conjugated molecules and various physical and chemical properties has been attempted many times. The bathochromic effect, produced by methyl groups on the electronic absorption spectra of 1,2-benzanthracene was measured by Jones39 and revealed a partial correlation with the carcinogenicity, but a comprehensive review by Pullman and Pullman40 on the question of electronic spectra and carcinogenicity concluded that no correlation existed. Several other studies41 and reviews42-44 have revisited the question but none have irrefutably confirmed any relationship.
The carcinogenic properties of alkylating agents have been extensively investigated.3
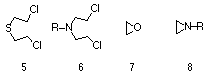
Almost one fifth of all chemicals identified as hazardous carcinogens to humans are identified as alkylating agents. Generally sulphur and nitrogen mustards, epoxides and aziridines, along with lactones, methansulphonyloxy and methanolamide derivatives are considered harmful (Figure 1-4). The chemical reactivity and metabolism of alkylating agents has focussed on the two alternative mechanisms of alkylation, namely unimolecular nucleophilic substitution (SN1) and bimolecular nucleophilic substitution (SN2). In the former, rate determining heterolysis provides a highly reactive electrophilic intermediate (usually a carbenium ion) that combines with a nucleophilic species such as DNA. It has been shown that aromatic nitrogen mustards usually undergo an SN1 process.45
In an SN2 process a carbenium ion is not generated but rather a nucleophilic compound reacts with an electrophilic reagent in a smooth one step process that passes through a transition state complex. The rate of reaction depends strongly on the nature of the nucleophile as well as the reactivity of the electrophilic substituent. PAH's were ultimately found to exert their carcinogenic effect through metabolic conversion to alkylating agents.
The role of chemicals in the process of carcinogenesis and mutagenesis has been based on conclusions of epidemiologists for over 3 decades. Higginson proposed that 60 to 90% of human cancers have environmental factors as their aetiologies. Through the early 1960's, cancer research like other scientific disciplines, was expanding rapidly and growing in sophistication. Numerous studies were being reported about the carcinogenic activity of many new classes of chemicals, such as alkylating agents,46,47 dialkylnitrosoamines,48 ethionine,49 and the pyrrolizidine alkaloids.50,51 These studies indicated that normal metabolic processes were intrinsically involved in the carcinogenic activity of many carcinogens.

Precarcinogenic compounds (procarcinogens) are inactive carcinogenic compounds that require metabolic activation through normal enzyme-catalysed reactions to provide a secondary or ultimate carcinogen (Scheme 1-1). Some precarcinogen (primary) compounds include alkylating agents 5-8, dimethyl sulphoxide and a substantial number of aromatic amines, nitrosoamines and PAH's. The actual number of stages in the metabolic activation process is dependent on the precarcinogen and the type of promoting agents or co-carcinogens. Co-carcinogens are considered to display little carcinogenic activity themselves, but serve to enhance the effect of the precarcinogen or ultimate carcinogen. Ultimate carcinogens are generally electrophilic agents that have the potential to interact directly with DNA (Scheme 1-1(iii)), however natural enzymatic defence processes can deactivate either the precarcinogen or active carcinogenic form to an inactive metabolite that is excreted (Scheme 1-1(v-vi)). A study of ultimate carcinogens has proven difficult because of the inherently high toxicity levels and generally fast rates of reactions, however much evidence has been obtained by investigation of the excretion products which gives an indication of the structure of the ultimate carcinogens and their potential chemical reactivity towards nucleophilic centres.8
The structure of the metabolites found in the faeces and urine of animals such as rats, mice and rabbits which have been subjected to injection of alternant hydrocarbons such as naphthalene, phenanthrene, benzopyrene, 1,2,5,6-dibenzanthracene and the like, have been thoroughly studied. In the case of non-carcinogenic naphthalene (as well as phenanthrene and anthracene), metabolism provided phenolic and dihydrodiolic compounds,52-55 which were thought to form from an epoxide followed by perhydroxylation and then dehydration (Figure 1-5).56

The sheer number and complexity of molecules that arise from the metabolism of simple PAH's was quickly realised by cancer researchers. For example, while the monohydroxylation of benzene affords phenol, the hydroxylation of 1,2,5,6-dibenzanthracene (a carcinogen) forms seven possible derivatives. The large number of possible reaction products has made the identification of isolated metabolites tedious and difficult. However, given these hurdles much work was done4,38 including further studies of the metabolysis of anthracene and benzpyrene by Berenblum et al,57,58 Weigert59 and Harper,60 along with many other researchers.
One occupation particularly affected with high rates of urinary cancers was the dyestuffs industry. As early as 1895, Rehn had recognised that there was an occupational link between bladder cancer and men who worked in a magenta dyestuffs factory. In the following decades several studies in other countries confirmed the hazardous nature of aromatic amines and the incidence of bladder cancer.61 Indeed, aromatic amines have since proved to be a highly suitable group of carcinogens for investigation of the mechanism of carcinogenesis because they induce tumours in a wide variety of tissues.
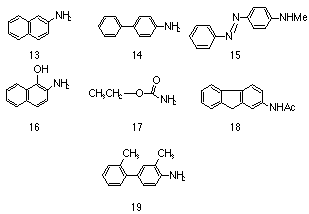
A commercially available quantity of pure 2-naphthylamine 13 was found by Hueper62 et al to induce both malignant and benign tumours in the bladder of 13 out of 16 dogs when administered orally and applied to the skin. These lesions were similar to those found in workmen employed in the chemical industry. Similar incidences of bladder cancer were induced from both partially and rigorously purified amines which indicated that, in the case for the amine at least, the amine and not some impurity in it was the effective carcinogen.
The method of administration of the amine and the influence of the strain of the test animal was quickly identified as an unknown variable4 but little work was done in ensuring a consistent and methodical approach to determining the relative mutagenicity of new compounds.
Strong evidence was found that the manufacture of 4-amino-biphenyl 14 similarly induced higher levels of bladder cancer in workers as did N-methyl-4-aminoazobenzene 15 and 2-acetylaminofluorene 18 but the relative potency of these compounds could not be quantified absolutely. The usual method for testing the efficacy of the amine was either administering a large quantity to animals either orally or through painting their skin, although the latter is less satisfactory because relatively little of the compound can be administered diminishing the yield of tumours and increasing the latency period. Furthermore, different strains of animals respond differently to aromatic amines4,63 compounding the difficulty in correlating results between laboratories. However in important work, Miller and Miller64,65 tested 2-acetylaminofluorene 18 and 4-acetamidobiphenyl under controlled conditions on one strain of rat and found preliminary correlations between the site of attack and chemical structure.
In the pre-World War II years, 2-acetylaminofluorene 18 was considered a promising insecticide until Wilson, DeEds and Cox66 found that it induced a wide variety of tumours in rats. Fortunately this early work hastened other studies on this compound, which have over time become so vast as to be impractical to review. Suffice to say that 18 is now regarded as a most ubiquitous carcinogen that has been found to induce tumours in nearly all species of animals to which it has been administered.
It soon became clear to researchers that the presence of certain structural features promoted carcinogenic activity from aromatic amines:4
The influence of the para substituent on activity is distinctly shown by the relative mutagenicities of 1-naphthylamine and 2-naphthylamine; the latter is considered a potent carcinogen whereas the former failed to induce a statistically significant greater level of tumours in rats even over a large latent period of 8 years.67 The influence of the positioning of the amino group in determining potency is reflected in the relative mutagenicity levels of aniline and 4-aminobiphenyl 14. The mutagenicity level of the former has been tested several times and has not been conclusively identified as a serious health hazard, whereas 14 is a potent carcinogen.
While not all aromatic amines are potent carcinogens, many polycyclic amines in which the position para to the amino group is blocked tend to exhibit carcinogenic behaviour. However factors other than a complex aromatic system and a blocked para position are important. Carcinogenic activity is, for example, enhanced when methyl substituents68 are present ortho to the amino group. The differences in carcinogenic activity between 2,3-dimethyl-4-aminobiphenyl 19, and the unsubstituted 4-aminobiphenyl 14 are well documented,4,68 the former being significantly more potent in rats.
The propensity for aromatic amine and amide carcinogens to induce tumours in tissues distant from the site of application indicated that these compounds require metabolic activation. When poisonous substances enter the living system, enzymatic processes begin to detoxify the chemical by converting them to water soluble metabolites. This process is typically carried out by a mixed-function oxidase which converts compounds into the more polar, hydroxylated form which is more readily excreted. An example of this is the cytochrome P-450 species which is found in all forms of life and is used to oxidise chemicals.
In 1951, Bonser, Clayson and Jull69 reported that 2-naphthylamine 13 was converted to 2-amino-1-naphthol 16 in dogs, mice, rats and rabbits and that the high occurrence of tumours in dog bladders correlated with the much higher concentration of 2-amino-1-naphthol derivatives found in the urine. Whether the amine or the ortho-hydroxyamine was the active metabolite was not unequivocally determined but further experiments70,71 involving direct application of substrates through bladder implantation72 indicated that the hydroxylated derivative was possibly carcinogenic. Indeed, the carcinogenic activity of these hydroxylated amine derivatives was the subject of a number of further studies4 which concluded that the ortho-hydroxyamine was generally found to be the most active derivative for a number of aromatic amines. Clayson et al73 showed that there was a correlation between the proportion of the amine converted to derivatives of 2-amino-1-naphthol 16 and to the carcinogenicity to the dog. However the efficacy of ortho-hydroxyamines to induce carcinogens appears to be subject to still unknown differences in the susceptibility of the species being investigated.
Several attempts74,75 have been undertaken to the determine if ortho-hydroxyamines are concentrated in the tumour site, but these studies have produced mixed results.
Amines can be metabolised to acetamides (R.NH.COCH3) through the action of acetylating enzymes which are present to varying degrees in a number of species. These processes are considered important as hydroxylation occurs at different positions for amines and acetamides. Nitrogen hydroxylated derivatives of aromatic amines conjugate with sulphuric acid and glucosiduronic acid and are excreted via the urine.
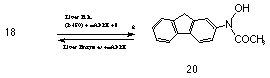
The importance of N-hydroxyamine derivatives in carcinogenesis was revealed by Cramer, Miller and Miller76 in 1960 with the isolation of N-hydroxy-2-acetylaminofluorene 20 from the urine of rats that had been administered 2-acetylaminofluorene 18.77 Long term metabolic studies by Cramer revealed that a new urinary metabolite N-hydroxy-2-acetylaminofluorene 20 was formed from the application of 2-acetylaminofluorene. Indeed, N-hydroxy-2-acetylaminofluorene 20 was more carcinogenic and more active in a wider range of tissues than the parent amide in rodents. One of the crucial factors which prevents an amine from being a carcinogen appears to be related to the ability of the animal to N-hydroxylate a sufficient quantity of the amine. For example 2-acetylaminofluorene is not carcinogenic in guinea pigs, which have little or no ability to N-hydroxylate this amine, whereas N-hydroxy-2-acetylaminofluorene 20 does produce tumours.
N-hydroxy-2-acetylaminofluorene 20 is also converted to a weak electrophilic O-glucuronide derivative by hepatic microsomes.65,78
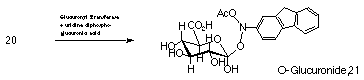
Cramer found that of the administered parent amide 20-50% is excreted in the urine and bile as O-glucuronide 21 (Scheme 1-3). As this metabolite is similarly excreted in large quantities by species that are less susceptible to tumours from this hydroxamic acid, it is not considered to be the major active metabolite responsible for carcinogenic activity and in vivo activity of N-hydroxy-2-acetylaminofluorene 20 in the rat.
Miller and Miller found that the direct intraperitoneal injection of N-hydroxy-2-acetylaminofluorene 20 lead to tumours similar to those found for the 2-acetylaminofluorene 18.79 Indeed in the case of rats and other rodents, N-hydroxy-2-acetylaminofluorene 20 proved to be the first known metabolite in which the N-hydroxylated derivative was more carcinogenic than the parent amide. These same authors proposed that N-hydroxylation of the amide could lead to compounds capable of combining with tissue proteins and thus causing cancer either through conversion of the N-hydroxyl derivative in vivo into an ortho-hydroxyamine, or rearrangement to other compounds that can combine directly with tissue. While DNA-, RNA- and protein bound derivatives were detected after application of both parent amide and N-hydroxy derivative in the liver, no reactivity was detected for the N-hydroxy derivative in vitro. Thus N-hydroxy-2-acetylaminofluorene 20 is a proximate carcinogen and further steps are required for the formation of the ultimate carcinogen.
Establishment of these enzymatic processes acting on aryl amines and the determination of the DNA fragments was undertaken by a wide range of researchers on several continents. Elucidation of the type and properties of ultimate carcinogens which form the resulting electrophilic metabolites has been actively studied, with several DNA residues being isolated. One important track has been the investigations involving the carcinogenicity of 2-acetylaminofluorene 18 and N-hydroxy-2-acetylaminofluorene 20.
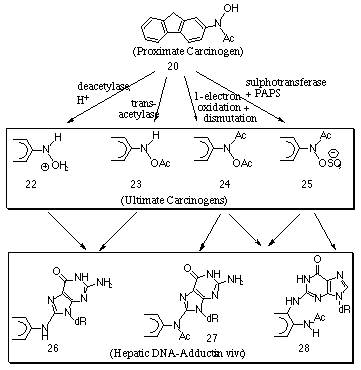
Following cytochrome P-450 oxidation of 2-acetylaminofluorene, the proximate carcinogen 20 is acted upon by a rich variety of metabolic processes which convert it to a number of reactive metabolites that subsequently bind with DNA (Scheme 1-4).
Both acetylated and non-acetylated aminofluorene adducts have been isolated from the livers of rats treated with N-hydroxy-2-acetylaminofluorene 20. The acetylated adducts, 27 and 28 were presumed to be formed from the N-hydroxy-2-acetylaminofluorene while the non-acetylated residues are presumably derived from the esters of N-hydroxy-2-aminofluorene or from the reaction of the O-glucuronide 21. The major adducts are those in which the substitution occurs at the C-8 of guanine.
Deacetylation and protonation forms the intermediate 22 which can lead to adducts without an N-acetyl group. Similar adducts can be formed after transacetylation to 23. Irving78 found that 70% of the isolated fluorene residues of rat hepatic ribosomal RNA retained the acetyl group whereas the majority of fluorene residues in the rat hepatic DNA do not. Scheme 1-4 displays some possible reaction processes in which the acetyl group is lost.
A one-electron oxidation of the N-hydroxy compound to a nitroxide free radical and the subsequent dismutation of the free radical can lead to the formation of N-acetoxy-2-acetylaminofluorene 24, and this process has been found to be catalysed by peroxidases in vitro.65 The acetyltransferase is of special importance in view of the wide range of tissues in which activity occurs and the very high reactivity of the product 24. This powerful electrophilic agent binds with guanine residues to form the adducts 27 and 28.
DeBraun and co-workers80 identified 25, the sulphuric acid ester of N-hydroxy-2-acetylaminofluorene, as a principal active metabolite in rat liver. Indeed the acute hepatic carcinogenesis of the amide increased with the administration of large doses of sodium sulphate. A strong correlation between the hepatic sulphanotransferase activity and the carcinogenicity of the applied compound was found. They reported that the male rat has a higher level of sulphanotransferase activity and a greater susceptibility to the carcinogenic activity of N-hydroxy-2-acetylaminofluorene 20. The correspondence between the in vitro sulphotransferase and the in vitro reactivity suggested that 2-acetylaminofluorene-N-sulphonate 25 was a major reactive form from the metabolism of 2-acetylaminofluorene. Subsequently, Westra et al81 found that DNA exposed to esters of N-hydroxy-2-acetylaminofluorene 20 formed 3-(deoxyguanosin-N2-yl)-N-acetyl-2-aminofluorene 28 residues.
Alkylating agents typically react at the N-7 position of guanine whereas with esters of N-hydroxy-2-acetylaminofluorene the reaction site is at C-8. The actual mode of nucleophilic attack has not yet been unequivocally determined. Nucleophilic attack by nucleotides have recently been investigated by Boche82,83 and Novak84-86 who found support for SN2 reactions at nitrogen. Unimolecular solvolysis of N-acetyl and N-sulphatooxy arylamines in aqueous solutions at physiological pHs has also been investigated indicating formation of arylnitrenium ions,87-91(see Section 1.3.3). N-acetoxy-2-aminofluorene is also formed from the parent amide and the reaction with DNA has been postulated as proceeding via a nitrenium ion intermediate.61
Overall, N-hydroxy derivatives are proximate carcinogens and are obligatory intermediates in the carcinogenicity of aromatic amines, amides and nitro compounds. Esterification appears to be critical to carcinogenicity for at least some aromatic amines, notably 2-acetylaminofluorene. The esters of N-hydroxy-2-acetylaminofluorene are highly mutagenic towards DNA and have short half lives (in the range of minutes in water). The synthetic esters react readily with guanine residues in nucleosides, nucleotides or nucleic acids and to a limited extent with adenine receptors. Furthermore the esters of N-hydroxy-2-acetylaminofluorene react at the C-8 position of guanine in contrast to the attack of alkylating agents which attack at the N-7 position of guanine.
At present the goal of numerous researchers is to determine the mode of action by which aromatic amines are metabolised into electrophilic metabolites and the subsequent reaction mechanism by which they interact with DNA.